Buy Nebido Online | Best India Prices
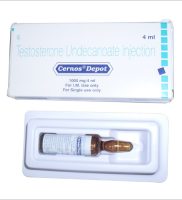
Testosterone Undecanoate (Cernos Depot) 1000mg/4ml
| Active substance | Testosterone Undecanoate |
| US Brand | Nebido |
| IN Brand | Cernos Depot |
| Manufacturing by | Sun Pharmaceutical |
| Strength | 1000mg/4ml |
| Form release | 1 ampoule |
| Shipping time | 7 – 18 days (Depending from the Country) |
| Availability & Order | through request form |
Our company offer to buy cheap Nebido alternative from India, it’s absoletly same active ingredient as Nebido, india brand name: Cernos Depot, you can order Nebido Generic from our company through request page, after that we will provide you with prices on Nebido analog 1000mg/4ml – 1 ampoule. We can ship our Nebido, to USA, UK, Australia.
Nebido contains testosterone, a male hormone, as the active ingredient. Nebido is injected into a muscle in your body. There it can be stored and gradually released over a period of time. Nebido is used in adult men for testosterone replacement to treat various health problems caused by a lack of testosterone (male hypogonadism). These should be confirmed by two separate blood testosterone measurements and also include clinical symptoms:
-
- impotence
- infertility
- low sex drive
- tiredness
- depressive moods
- bone loss caused by low hormone levels
You can order analog Nebido with cheap prices from India.
It is used in androgen replacement therapy primarily for the treatment of male hypogonadism, and has also been investigated for use as a male contraceptive or as hormone replacement therapy in transgender men. Unlike other testosterone esters, testosterone undecanoate is available in both oral and intramuscular formulations.
Posology
One ampoule / vial of Nebido (corresponding to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation.
Start of treatment
Serum testosterone levels should be measured before start and during initiation of treatment. Depending on serum testosterone levels and clinical symptoms, the first injection interval may be reduced to a minimum of 6 weeks as compared to the recommended range of 10 to 14 weeks for maintenance. With this loading dose, sufficient steady state testosterone levels may be achieved more rapidly.
Maintenance and individualisation of treatment
The injection interval should be within the recommended range of 10 to 14 weeks. Careful monitoring of serum testosterone levels is required during maintenance of treatment. It is advisable to measure testosterone serum levels regularly. Measurements should be performed at the end of an injection interval and clinical symptoms considered. These serum levels should be within the lower third of the normal range. Serum levels below normal range would indicate the need for a shorter injection interval. In case of high serum levels an extension of the injection interval may be considered.
Medicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that are known to be associated with this medicine. Just because a side effect is stated here, it does not mean that all people using this medicine will experience that or any side effect.
-
-
- Reaction at the injection site.
- Prostate problems, such as growth of the prostate gland, increased PSA levels and prostate cancer (see warning section above).
- Headache.
- Feeling sick and vomiting.
- Mood changes.
- Depression.
- Nervousness, anxiety or irritability.
- Change in sex drive.
- Breast pain, enlargement of the breasts.
- Persistent painful erection of the penis (priapism). Tell your doctor straight away if you experience this.
- Decreased sperm count.
- Swelling caused by fluid retention (oedema).
- Increased blood pressure (hypertension).
- Increased levels of red blood cells and haemoglobin in the blood (polycythaemia).
- Weight gain.
- Pain in the muscle or joints.
- Male-pattern hair loss.
- Acne.
- Itching.
- Raised cholesterol levels.
- Jaundice.
- Liver tumours.
- Premature sexual development if used in boys who haven’t reached puberty.
- Premature closure of the ends of bones, causing stunted growth if used in boys who haven’t reached puberty.
-
The side effects listed above may not include all of the side effects reported by the medicine’s manufacturer. For more information about any other possible risks associated with this medicine, please read the information provided with the medicine or consult your doctor or pharmacist.